Order: Perissodactyla
- Black Rhinoceros
- Domestic Horse
- Greater Indian Rhinoceros
- Kiang
- Malayan Tapir
- Somali Wild Ass
- Southern White Rhinoceros
- Zebra Species
Southern White Rhinoceros (or square-lipped rhinoceros)Ceratotherium simum simumOrder: Perissodactyla |
 |
White rhinoceros in the San Diego Zoo's Wild Animal Park. |
3) Implantation Early implantational stages were depicted by the sonographic study of Radcliffe et al. (1997). The earliest stage shown occurred 15 days after ovulation, with embryonic definition visible on day 23, and heartbeat visible on day 26. The exact time of implantation, however, has not yet been defined. The placenta is diffusely villous, with larger streak-like areas of villous absence. This is one reason for the organ to be called "Placenta villosa diffusa incompleta". These bare areas are also referred to as "streets (Strassen)". The organ implants in both uterine horns, with the fetus located mostly in one horn, and the placenta extending to both sides. |
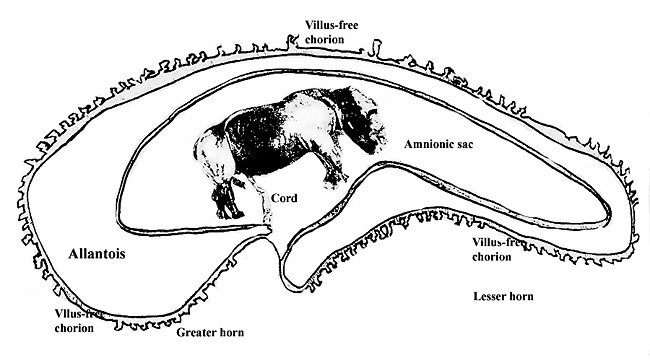 |
Schematic representation of rhinoceros placentation. |
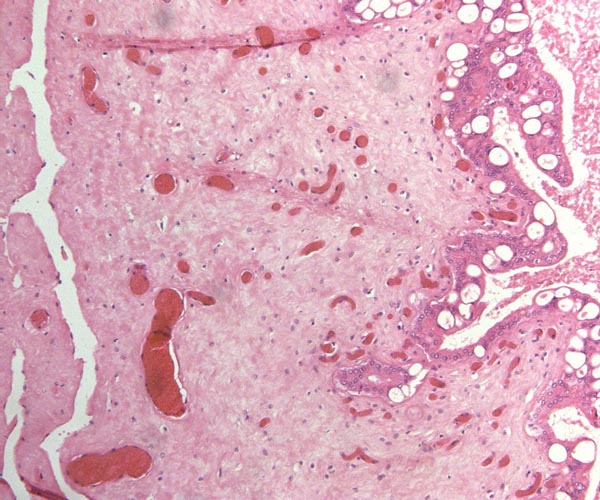
4) General characteristics of placenta The placentas are very large and very thin. One weighing 7,600 g is depicted here. It measured 190 cm in length, 100 cm in greatest width and 50 cm in smallest diameter. It was 2 mm in thickness. Another placenta examined by me weighed 6,150 g, measured 210x56 cm, was typically thin and had an extremely short cord with four vessels and allantoic duct. The placenta of a term Indian rhinoceros weighed 5,300 g, with an additional 950 g of amnionic and allantoic membranes. It measured 230 cm in greatest width. These are diffuse placenta without cotyledons, and with an epitheliochorial barrier character. Many placentas have on their maternal surfaces band-like connective tissue areas that are bare of villi. They usually follow the lesser curvature of the placenta and have been referred to as "Strassen" (streets) in the Suisse literature. A very young gestation has recently become available for study. It occurred in an old (35 years or more) female that has had 15 young before and died from intestinal volvulus. The macroscopic pictures are shown next. |
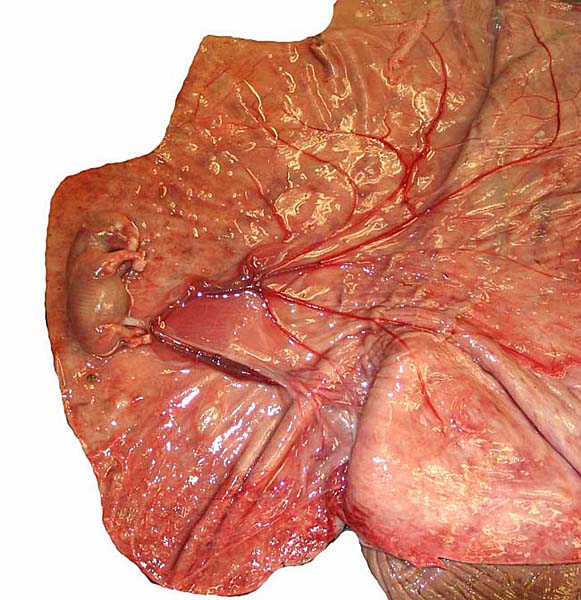 |
Early stage of white rhino placentation. Embryo is 35 g and is still within the amnion. |
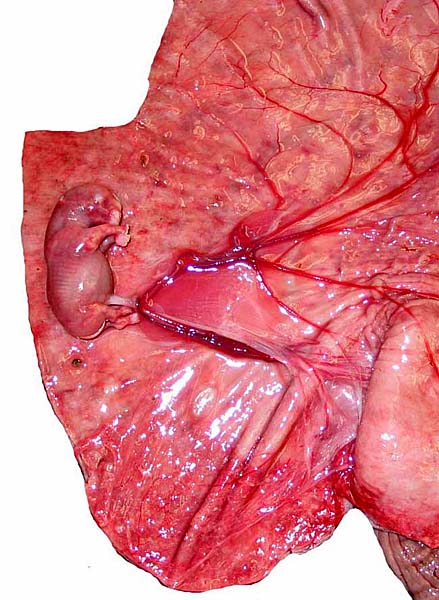 |
The amnion has been removed and a very short umbilical cord can be seen whose vessels branch into two sections for future occupation of other uterine horn. |
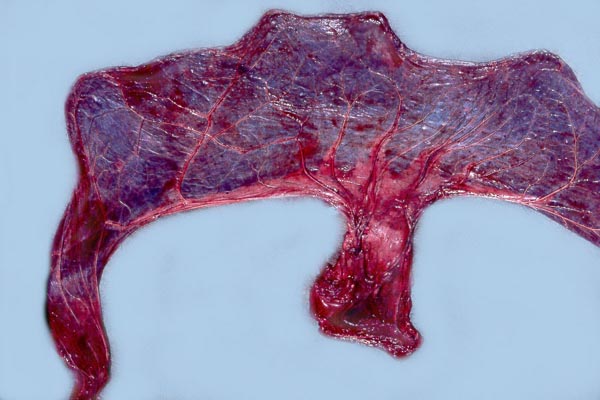 |
White rhinoceros, delivered placenta, fetal side. |
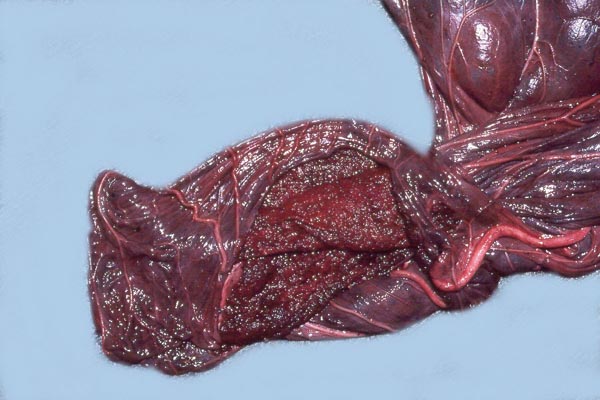 |
Maternal aspect of white rhinoceros placenta (center). |
The cord is relatively short and there is a large, heavily vascularized allantoic sac. Hippomanes (yellow/brown) are composed of eosinophilic debris with or without crystalline inclusions (180 g in an Indian rhino). In addition, there are numerous round, yellowish squamous patches projecting on the amnionic surface of some black and Indian rhinoceros placentas; they measure 0.5-1 cm. |
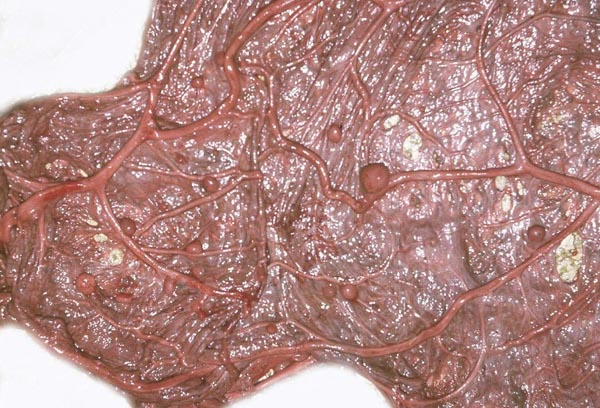 |
Amnionic surface of Indian rhinoceros placenta with round nodules. |
Leaf-like and folded villi were alluded to by Ludwig et al. (1965), representing thinner and others, taller epithelial structures. Histochemical reactions were possible that showed minor differences (Ludwig & Müller, 1965). Occasional binucleated trophoblastic cells have been described, but there is no uterine invasion by trophoblast. The electronmicroscopic study by Ludwig & Villiger (1965) identified similarities to equine placentas; numerous trophoblastic transport vesicles were seen. The allantoic sac is anchored by thin connective strands to the chorion (Dolinar et al., 1965). While the amnionic epithelium is very thin and flat, the allantoic sac is lined by cuboidal to columnar epithelium. The allantois is diffusely vascularized. |
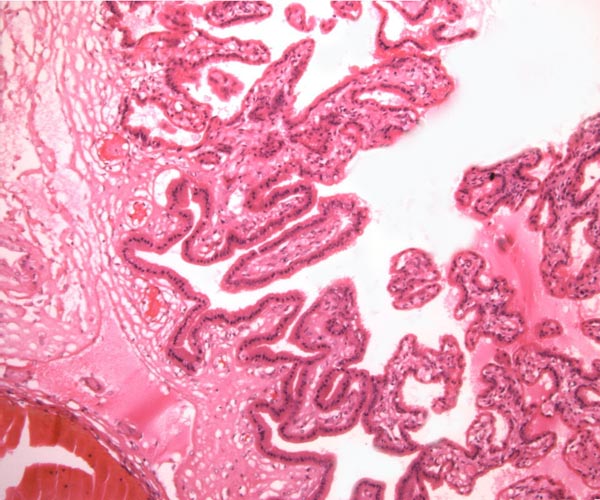 |
Villi attached to chorion (left) of white rhinoceros. |
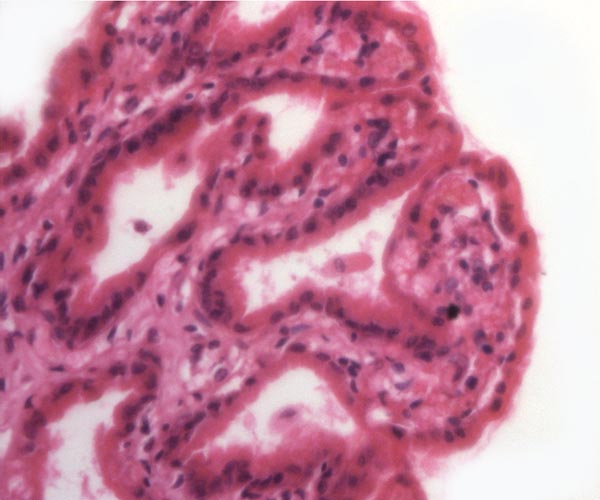 |
Higher magnification of white rhinoceros villi. |
|
5) Details of barrier structure 6) Umbilical cord |
||||||||||||||||||||||||||||||||||||||||||||
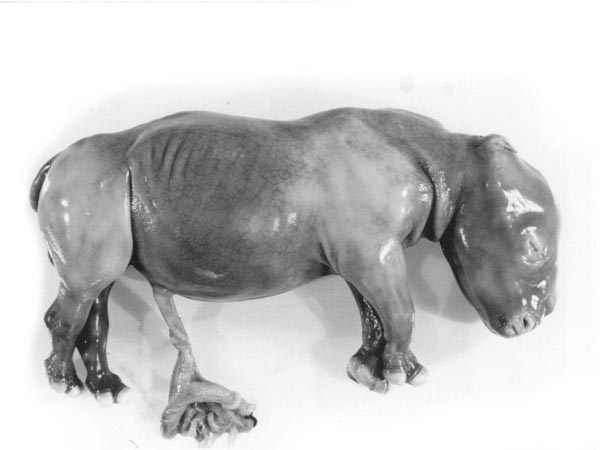 |
Fetus of Southern white rhinoceros with small portion of placenta attached. Note the short length of the umbilical cord. |
A remarkably long umbilical cord (57.8 cm) in an Indian rhinoceros was described by Dolinar et al. (1965). The umbilical cord has 4 large umbilical vessels and a very large number of small vessels that provide the circulation for the allantois. It also has a large allantoic duct. A vitelline duct is absent. The surface of umbilical cords has squamous plaques. |
 |
Portion of rhinoceros umbilical cord. Large vessel at left; numerous small allantoic vessels are evident. |
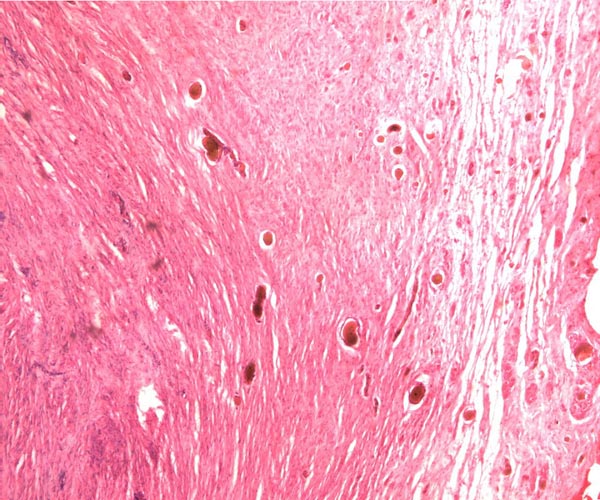
7) Uteroplacental circulation No descriptions are available. 8) Extraplacental membranes |
 |
Amnion at left (flat epithelium) allantois right (columnar epithelium). |
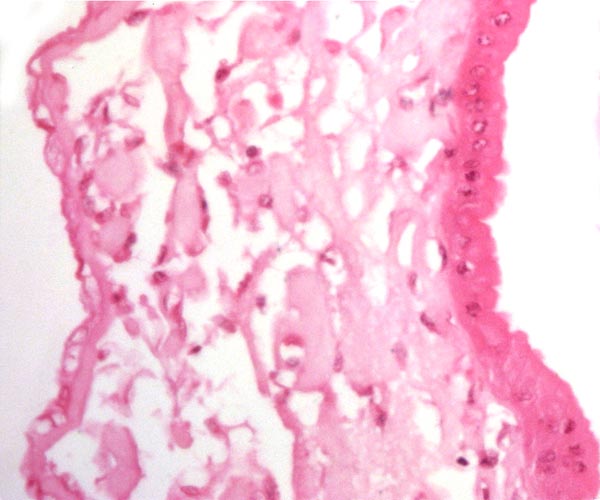
9) Trophoblast external to barrier There is no trophoblast beyond the villous structures. 10) Endometrium 11) Various features 12) Endocrinology
14) Immunology 15) Pathological features |
||||
 |
Indian rhinoceros chorionic surface with abnormally vacuolated trophoblast at right, overlying endometrial debris. |
16) Physiological data Whatever relevant physiologic data are available have been provided in review form by Silberman & Fulton (1979), and as bibliography by Miller (1983). 17) Other resources None are available of the severely endangered Javan rhinoceros. References Benirschke, K. and Lowenstine, L.J.: The placenta of the rhinocerotidae. Verh. Ber. Erkr. Zootiere (Dresden). 37:15-23, 1995. Carlstead, K., Fraser, J., Bennett, C. and Kleiman, D.G.: Black rhinoceros (Diceros bicornis) in U.S. Zoos: II. Behavior, breeding success, and mortality in relation to housing facilities. Zoo Biol. 18:35-52, 1999. Chapin, H., Malecek, A.C., Miller, R.E., Bell, C.E., Gray, L.S. and Hunter, V.L.: Acute intravascular hemolytic anemia in the black rhinoceros: Hematologic and immunohematologic observations. Amer. J. Vet. Med. 47:1313-1320, 1986. Dolinar, Z.J., Ludwig, K.S. und Müller, E.: Ein weiterer Beitrag zur Kenntnis der Placenten der Ordnung Perissodactyla: Zwei Geburtsplacenten des Indischen Panzernashorns. (Rhinoceros unicornis L.). Acta Anat. 61:331-354, 1965. George, M. Jr., Chemnick, L.G., Cisova, D., Gabrisova, E., Stratil, A. and Ryder, O.A.: Genetic differentiation of white rhinoceros subspecies: diagnostic differences in mitochondrial DNA and serum proteins. In, Proc. Intern. Conference on Rhinoceros Biology and Conservation, San Diego, CA 1991, pp. 105-113. Groves, C.P.: Phylogeny of the living species of Rhinoceros. Z. zool. System. Evol. 21:293-313, 1983. Harley, E.H. and O'Ryan, C.: Molecular genetic studies of southern African rhinoceros. In, Proc. Intern. Conference on Rhinoceros Biology and Conservation, San Diego, CA 1991, pp. 101-104. Heinichen, I.G.: Karyological studies on southern African perissodactyla. Kodoe 13:51-108, 1970. Houck, M.L., Ryder, O.A., Váhala, J., Kock, R.A. and Oosterhuis, J.E.: Diploid chromosome number and chromosomal variation in the white rhinoceros (Ceratotherium simum). J. Hered. 85:30-34, 1994. Hungerford, D.A., Chandra, H.S. and Snyder, R.L.: Somatic chromosomes of a black rhinoceros (Diceros bicornis Gray 1821). Amer. Naturalist 101:357-358, 1967. Lang, E.M.: Geburt eines Panzernashorns, Rhinoceros unicornis, im Zoologischen Garten Basel. Säugetierk. Mitt. 5:69-70, 1957. Lang, E.M.: Einige biologische Daten vom Panzernashorn (Rhinoceros unicornis). Rev. Suisse Zool. Genève 74:603-607, 1967. Laurie, W,A., Lang, E.M. and Groves, C.P.: Rhinoceros unicornis. In, Mammalian Species # 211, pp.1-6, 1983. Amer. Soc. Mammalogy. Ludwig, K.S.: Zur Kenntnis der Geburtsplacenten der Ordnung Perissodactyla. Acta Anat. 49:154-167, 1962. Ludwig, K.S. und Müller, E.: Zur Histochemie der Placenta des Panzernashorns (Rhinoceros unicornis L.). Acta Anat. Suppl. 115:155-159, 1965. Mereniender, A.M., Woodruff, D.S., Ryder, O.A., Kock, R. and Váhala, J.: Allozyme variation and differentiation in African and Indian rhinoceroses. J. Hered. 80:377-382, 1989. Miller, R.E. and Boever, W.J.: Fatal hemolytic anemia in the black rhinoceros: Case report and a survey. J.A.V.M.A. 181:1228-1231, 1982. Morales, J.C., Andau, P.M., Supriatna, J., Zainuddi, Z.Z. and Melnick, D.J.: Mitochondrial DNA variability and conservation genetics of the Sumatran rhinoceros. Conserv. Biol. 11:539-543, 1997. Paglin, D.E., Valentine, W.N., Miller, R.E., Nakatani, M. and Brockway, R.A.: Acute intravascular hemolysis in the black rhinoceros: Erythrocyte enzymes and metabolic intermediates. Amer. J. Vet. Res. 47:1321-1325, 1986. Patton, L., Czekala, N. and Lance, V.: Workshop on problems associated with the low rate of reproduction among captive-born female Southern White Rhinoceros (Ceratotherium simum simum). Zoological Society of San Diego, 1998. Radcliffe, R.W., Czekala, N.M. and Osofsky, S.A.: Technical Report. Combined serial ultrasonography and fecal progestin analysis for reproductive evaluation of the female white rhinoceros (Ceratotherium simum simum): Preliminary results. Zoo Biol. 16:445-456, 1997. Schaller, K. and Pilaski, J.: Pocken bei Breitmaul-nashörnern (Ceratotherium s. simum) im Zoologischen Garten Münster. Zool. Garten 49:169-184, 1979. Silberman, M.S. and Fulton, R.B.: Medical problems of captive and wild rhinoceros - a review of the literature and personal experiences. J. Zoo Anim. Med. 10:6-16, 1979. |
Various Rhinoceroses |
 |
Sumatran rhinoceros at Copenhagen Zoo. |
 |
Indian Rhinoceros at San Diego Wild Animal Park. |
 |
Black rhinoceros with young at San Diego Wild Animal Park. |
 |
Southern White Rhinoceros at San Diego Wild Animal Park. |