14) Immunology
I know of no immunological studies on sable antelopes.
15) Pathological features
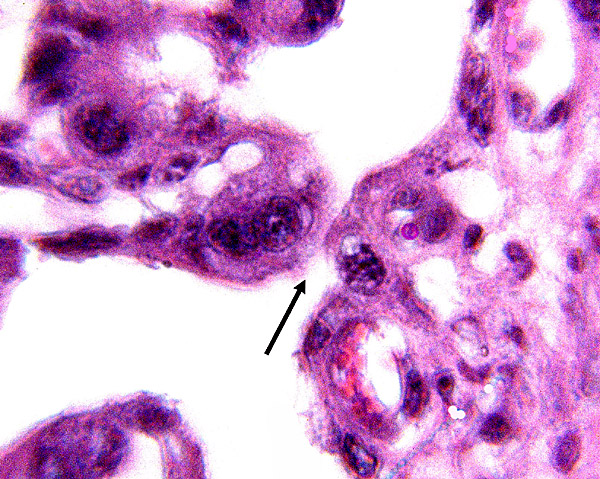
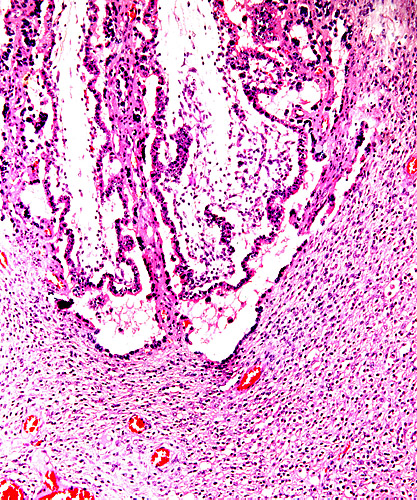
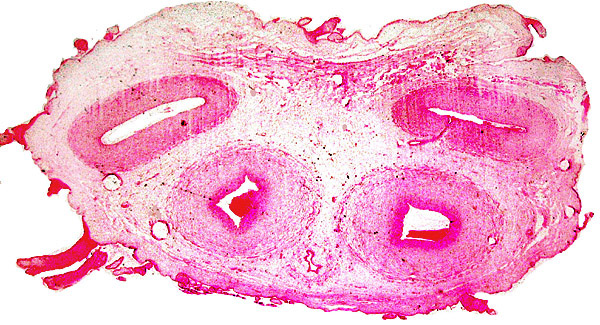
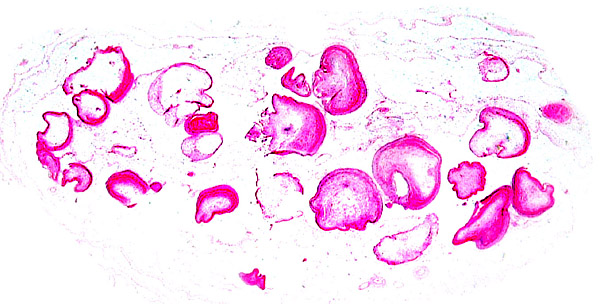
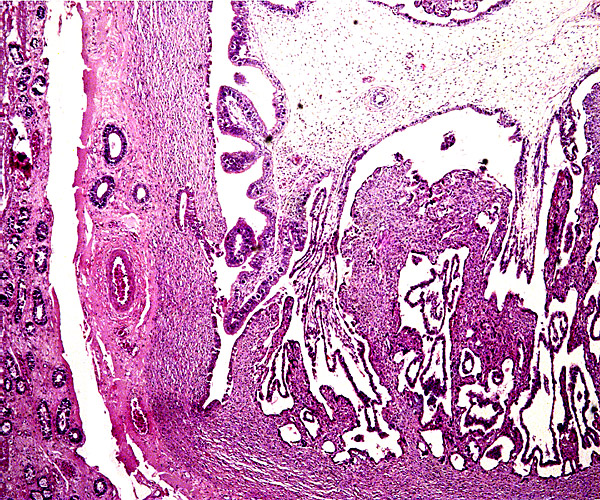
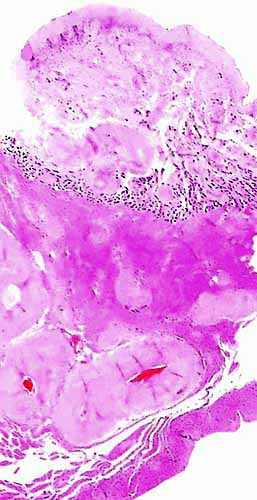
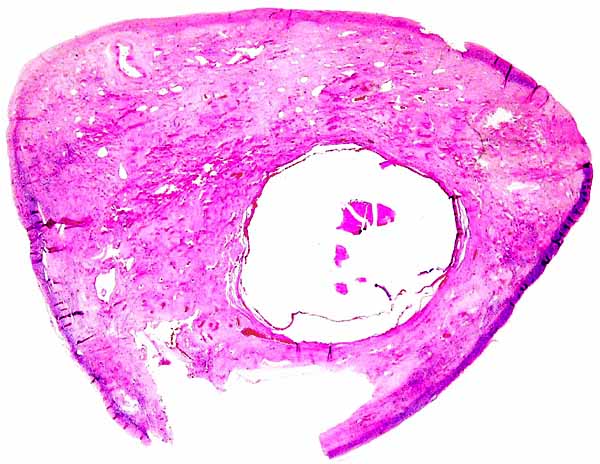
Griner (1983) found neonatal mortality (and stillbirths) the most important factors in his large series of autopsies at San Diego Zoo. Heuschele et al. (1984) found neutralizing antibodies to the virus causing malignant catarrhal fever in sable antelopes.
16) Physiologic data
Numerous biomedical parameters were studied in 35 neonates by Ferrell et al. (2001).
17) Other resources
There are numerous cell lines in the "Frozen Zoo" of CRESat the San Diego Zoo that can be made available by contacting Dr. O. Ryder at:oryder@ucsd.edu
18) Other remarks - What additional Information is needed?
There is no concrete information on the length of umbilical cords, no electronmicroscopy has been done, and there are no data on early implantations nor have endocrine parameters during pregnancy been published.
Acknowledgement
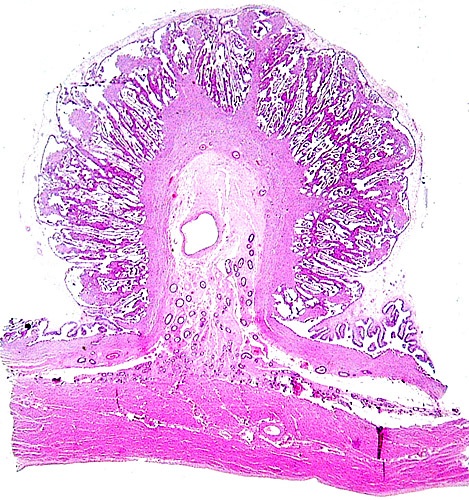
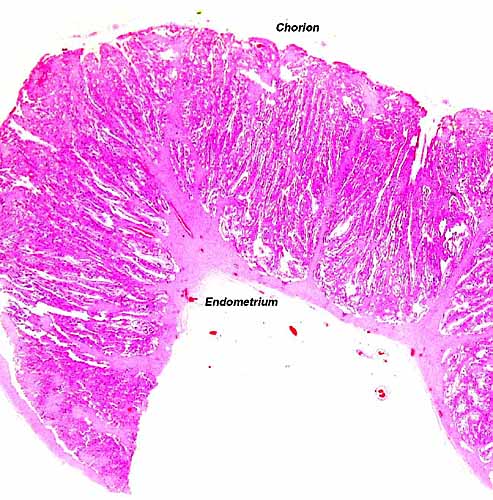
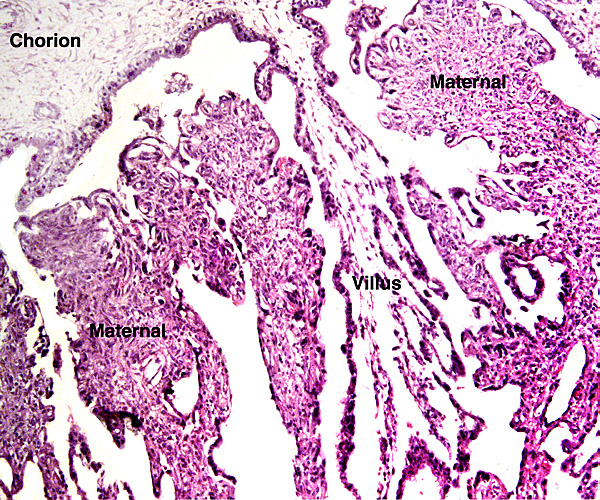
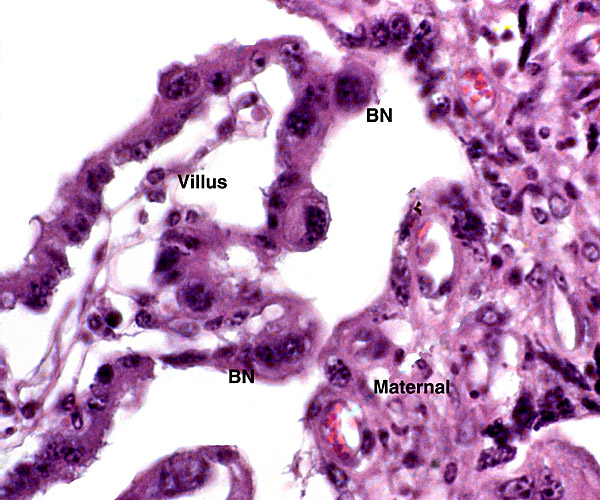
The animal photograph in this chapter comes from the Zoological Society of San Diego. The best-preserved sections of placental tissue were kindly donated by Dr. Petr Hradecky.
References
Blaine, G.: Notes on the zebras and some antelopes of Angola. Proc. Zool. Soc. London # 22:317-339, 1922.
Buechner, H.K., Stroman, H.R. and Xanten, W.A.: Breeding behaviour of Sable antelope Hippotragus niger in captivity. Intern. Zoo Yearb. 14:133-137, 1974.
Claro, F., Hayes, H. and Cribiu, E.P.: The R- and G-bands of the sable antelope (Hippotragus niger). J. Hered. 84:481-484, 1993.
Ferrell, S.T., Radcliffe, R.W., Marsh, R., Thurman, C.B., Cartwright, C.M., de Maar, T.W.J., Blumer, E.S., Spevak, E. and Osofsky, S.A.: Comparisons among selected neonatal biomedical parameters of four species of semi-free ranging hippotragini: Addax (Addax nasomaculatus), Scimitar-horned oryx (Oryx dammah), Arabian oryx (Oryx leucoryx), and sable antelope (Hippotragus niger). Zoo Biol. 20:47-54, 2001.
Fordyce-Boyer, R., Sanger, T., Loskutoff, N., Kumamoto, A.T., Johnston, L. and Armstrong, D.: Comparative cytogenetic study of the Roan and Sable antelope, Hippotragus equinus and Hippotragus niger. Applied Cytogenet. 21:189-191, 1995.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Groves, C.P.: A new subspecies of sable antelope, Hippotragus niger (Harris 1838). Rev. Zool. Afr. 97:821-828, 1983.
Hassanin, A. and Douzery, E.J.: The tribal radiations of the family Bovidae (Artiodactyla) and the evolution of the mitochondrial cytochrome b gene. Mol. Phylogenet. Evol. 13:227-243, 1999.
Heuschele, W.P., Oosterhuis, J., Anderson, M.P., Swansen, M. and Fletcher, H.R.: Malignant catarrhal fever in wild ruminants. Chapter 25 (pp. 296-308) In, One Medicine; O.A. Ryder and M. L. Byrd, eds. Springer-Verlag, New York, 1984.
Hradecky, P.: Placental morphology in African antelopes and giraffes. Theriogenology 20:725-734, 1983.
Hradecky, P.: Composition of the fetal fluids in African antelopes. Theriogenology
Hradecky, P., Mossman, H.W. and Stott, G.G.: Comparative histology of antelope placentomes. Theriogenology 29:693-6729, 1988a.
Hradecky, P., Mossman, H.W. and Stott, G.G.: Comparative development of ruminant placentomes. Theriogenology 29:715-729, 1988b.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. 3: Folio 142, 1969.
Jones, M.L.: Longevity of ungulates in captivity. Int. Zoo Yearb. 32:159-169, 1993.
Matthee, C.A. and Davis, S.K.: Molecular insights into the evolution of the family Bovidae: A nuclear DNA perspective. Mol. Biol. Evol. 18:1220-1230, 2001.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa. The Lammergeyer 16:1-89, 1972.
Mohr, E.: Der Blaubock. Hippotragus leucophaeus (Pallas, 1766). Eine Dokumentation Paul Parey, Hamburg, 1967.
Mossman, A.S. and Mossman, H.W.: Ovulation, implantation, and fetal sex ratio in impala. Science 137:869, 1962.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Wilson, D.E. and Hirst, S.M.: Ecology and factors limiting Roan andn Sable antelope populations in South Africa. Wildlife Monograph #54 in, J. Wildl. Management 41:1-111, 1977.
Wurster, D.H. and Benirschke, K.: Chromosome studies in the superfamily Bovoidea. Chromosoma 25:152-171, 1968.
|